Device description and product specification, i...
Accredited Consultants Private Limited 2023-06-27T04:38:34 2023-06-27T04:38:34
Accredited Consultants Private Limited 
Device description and product specification, i...

Currently it only shows your basic business info. Start adding relevant business details such as description, images and products or services to gain your customers attention by using Boost 360 android app / iOS App / web portal.











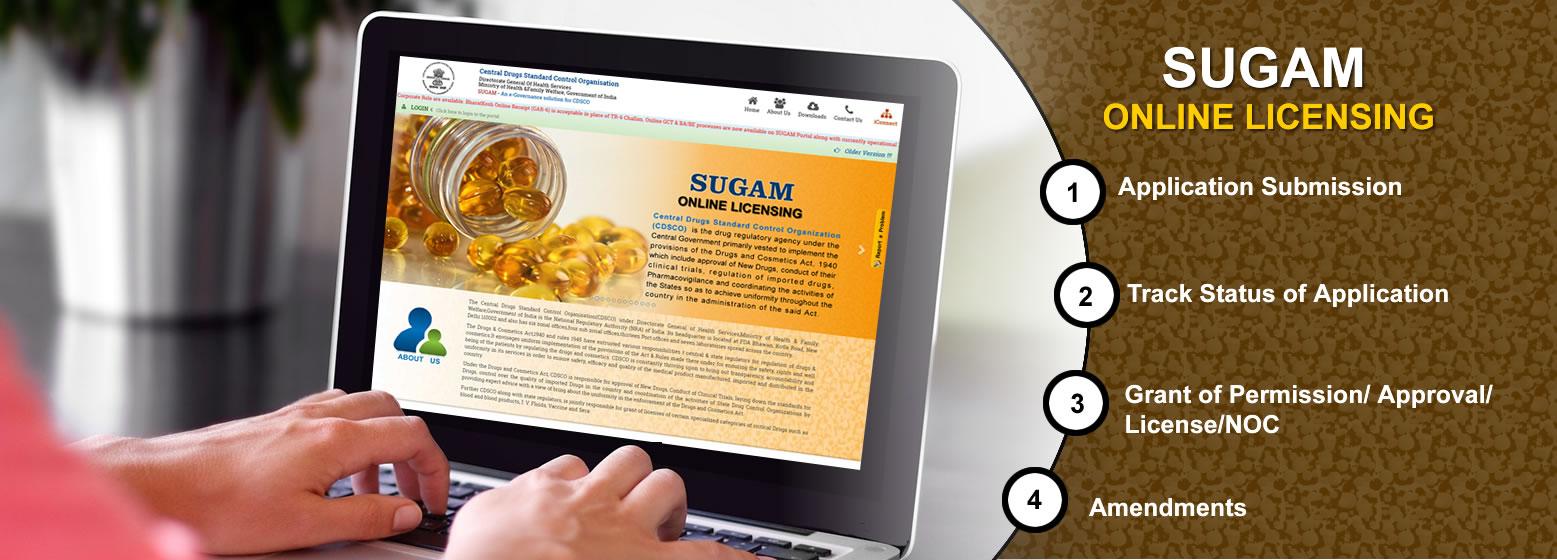


No services available for booking.
Select Staff

AnyBody
Appointment date & time
Sunday, 7 Aug, 6:00 PM